Remote sensing monitoring of dynamic land cover types: the ten-day temporal scale change of major lakes in China from 2000 to 2010
1
2014
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
动态地表覆盖类型遥感监测: 中国主要湖泊面积2000—2010年间逐旬时间尺度消长
1
2014
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
1
1998
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
1
1998
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
The ecological role of water-column microbes in the sea
1
1983
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
China lake microbiome project
1
2017
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
中国湖泊微生物组研究
1
2017
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
Research progress on bacterial diversity and ecological function in lake water
1
2013
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
湖泊水体细菌多样性及其生态功能研究进展
1
2013
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
Composition and structure of bacterial community in Poyang Lake: a case study of Songmen Mountain
1
2015
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
鄱阳湖湖泊细菌群落组成及结构——以松门山为例
1
2015
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
Analysis of bacterial community structure in different spatial distribution in Songhua Lake
0
2013
松花湖水体中不同空间分布的细菌群落结构分析
0
2013
Bacterial diversity in Adirondack mountain lakes as revealed by 16S rRNA gene sequences
0
1997
Bacterial diversity of freshwater alpine Lake Puma Yumco on the Tibetan Plateau
0
2009
Diversity of culturable bacteria and characteristics of extracellular active substances produced in alpine lakes of Southwest China
1
2017
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
西南地区高山湖泊中可培养细菌多样性及其所产胞外活性物质的特性
1
2017
... 湖泊在生物圈、大气圈、岩石圈和水圈之间的物质能量循环中充当重要载体[1].青藏高原拥有全球海拔最高、面积最大、数量最多的高原湖群区,其湖泊总面积约占全国湖泊总面积的二分之一[2].湖泊微生物在湖泊生态系统中发挥着不可或缺的作用[3],开展湖泊微生物研究对于揭示湖泊生态系统对外界条件变化的响应规律及深入了解湖泊生态系统来说有着重要意义[4].细菌是湖泊生态系统中的重要一员,湖泊中细菌的数量庞大且物种丰富,它们在湖泊生态系统绝大多数生物活性元素的形态转化和地球化学循环中起着重要作用[5],近年来,不少专家学者已逐步对其开展了相关研究[6-10]. ...
Surface area variations of lakes in the Tibetan Plateau and their influencing factors
1
2017
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
青藏高原湖泊湖面变迁及影响因素
1
2017
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Microbial ecology in the Tibetan Plateau based on metagenomics
1
2020
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
基于宏基因组学的青藏高原微生物生态研究
1
2020
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Quantity, community structure and driving factors of carbon sequestration microorganisms in Nam Co
1
2019
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
纳木错湖水体固碳微生物数量、群落结构及其驱动因子
1
2019
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Microbial diversity and adaptability of saltwater lakes at different elevations on the Tibetan Plateau
1
2014
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
青藏高原不同海拔咸水湖微生物多样性及适应性特征
1
2014
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Bacterial community characteristics in Nam Co and its comparison with alpine lakes on Tibetan plateau
1
2008
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
青藏高原纳木错湖细菌群落特征及其与高山湖泊的对比
1
2008
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Isolation, identification and determination of antimicrobial activity of actinomycetes from lakes in Tibet
1
2020
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
西藏湖泊放线菌的分离鉴定及抗菌活性测定
1
2020
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
Seasonal variation of culturable microorganisms in lake water from different altitudes of Tibetan Plateau
1
2013
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
青藏高原不同海拔湖水中可培养微生物的季节性变化
1
2013
... 青藏高原是中国最大、世界平均海拔最高的高原,也是中国湖泊的主要分布区之一[11].纳木错是典型的青藏高原湖泊,孙丹[12]通过高通量测序等方法,揭示了纳木错表层湖水与岸边土壤环境中的微生物群落构成;刘金波等[13]用定量PCR和克隆文库方法,研究了纳木错湖水中cbb L ID基因丰度和固碳微生物群落组成,并分析了其与环境参数的关系;王鑫[14]运用宏基因组测序等技术研究了湖中微生物的物种组成、物种分布及物种多样性;刘晓波等[15]通过流式细胞计数等技术分析了纳木错水体中细菌的群落组成.然而关于纳木错湖泊可培养微生物的研究报道较少,潘文娟等[16]从纳木错沉积物中分离得到6株放线菌,填补了西藏湖泊放线菌资源的空白;张红光等[17]研究了纳木错水体可培养微生物丰度是否受海拔梯度造成的气候环境因素的影响,结果表明纳木错可培养微生物的数量与温度呈显著正相关.但关于纳木错水体可培养细菌群落多样性及其对水质理化指标的响应规律的研究鲜有报道,探究青藏高原湖水可培养细菌群落多样性,将有着重要的生态学意义. ...
A new understanding of the changes in water quantity and water quality of lakes on the Qinghai-Tibet Plateau
1
2017
... 纳木错(90°16′~91°03′ E,30°30′~30°55′ N)位于青藏高原,湖泊面积达2 020 km2[18],流域面积达10 610 km2,跨越范围89°21′~91°23′ E,29°56′~31°7′ N,流域周边的冰川融水会汇入纳木错湖泊[19],成为湖泊水体的重要来源之一. ...
青藏高原湖泊水量与水质变化的新认知
1
2017
... 纳木错(90°16′~91°03′ E,30°30′~30°55′ N)位于青藏高原,湖泊面积达2 020 km2[18],流域面积达10 610 km2,跨越范围89°21′~91°23′ E,29°56′~31°7′ N,流域周边的冰川融水会汇入纳木错湖泊[19],成为湖泊水体的重要来源之一. ...
Quantitative analysis of lake area change and its causes in Nam Co, Tibet from 1971 to 2004
1
2010
... 纳木错(90°16′~91°03′ E,30°30′~30°55′ N)位于青藏高原,湖泊面积达2 020 km2[18],流域面积达10 610 km2,跨越范围89°21′~91°23′ E,29°56′~31°7′ N,流域周边的冰川融水会汇入纳木错湖泊[19],成为湖泊水体的重要来源之一. ...
西藏纳木错1971—2004年湖泊面积变化及其原因的定量分析
1
2010
... 纳木错(90°16′~91°03′ E,30°30′~30°55′ N)位于青藏高原,湖泊面积达2 020 km2[18],流域面积达10 610 km2,跨越范围89°21′~91°23′ E,29°56′~31°7′ N,流域周边的冰川融水会汇入纳木错湖泊[19],成为湖泊水体的重要来源之一. ...
Characteristics of culturable bacterial community in coastal water of Nam Co in spring
7
2022
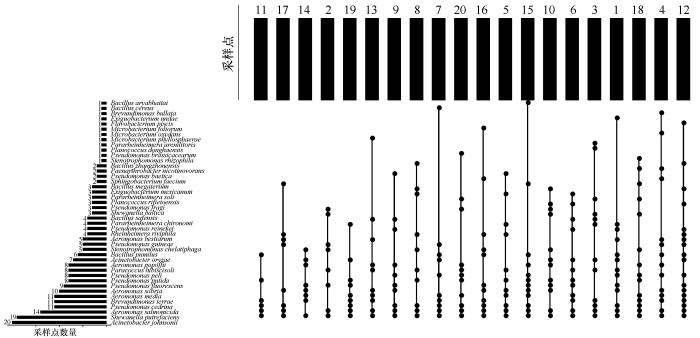
... 2020年夏季沿纳木错设20个样点采集水样,本研究区域由于条件限制,无法达到湖面,因此仅在沿岸区设置样点.其中2号样点在扎西半岛,5号样点与主湖间隔了一道天然河坝,10号样点在多加寺附近,11号样点采样时水中长有水草,15号样点湖岸边上有很多动物粪便,19号样点在观景台,20号样点在湖泊入口处(图1).用无菌采集器在距湖岸边约15 m、距水体表面约50 cm处进行水样采集[20].直接在采样地用多参数测试笔测定水样pH、温度、总溶解固体量、电导率和盐度,每个参数测定3次[21].每个样点共采集约7.5 L水样,平均装入3个无菌塑料桶.1份水样立即送至西藏博源环境检测有限公司测定水体理化因子,包括总氮、总磷、氨氮、化学需氧量与浊度;1份水样带回实验室立即分离其中的可培养细菌[20]. ...
... [20]. ...
... 细菌的分离培养采用直接涂布平板法与稀释涂布平板法相结合.将20个样点的水样使用移液枪分别取200 μL接种于固体培养基平板(牛肉膏蛋白胨培养基:牛肉膏10 g,蛋白胨10 g,氯化钠5 g,琼脂粉20 g,水1 L,pH 7.4~7.6)上,采用涂布器将水样涂布均匀后放入恒温箱中倒置培养,温度设置为28 ℃.其中菌落数过多的样点重新采用稀释涂布平板法进行分离培养(5号、10号、11号、15号和19号).每个处理设3个重复.待平板上长出清晰可见的菌落时依次进行计数、分离、纯化及保藏等步骤,详见文献[20]. ...
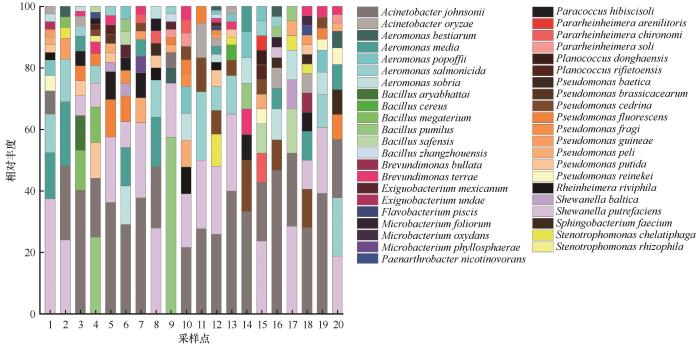
... 对供试菌株16S rDNA序列测序结果进行人工校对,去除引物及双峰序列后在GenBank数据库中进行比对,从而确定菌株的分类地位[20].将供试菌株的基因序列上传至GenBank核酸数据库,登录号为MW799879~MW799953,共75条序列.下载与代表菌株基因序列相似性高的菌株序列,使用MEGA 7.0软件中的邻接(neighbour-joining)法构建基于16S rDNA基因序列的系统发育树,其中检验次数设为1 000次[23],确定本研究中菌株的系统发育学地位[20]. ...
... [20]. ...
... 各项理化因子的测定详见文献[20]. ...
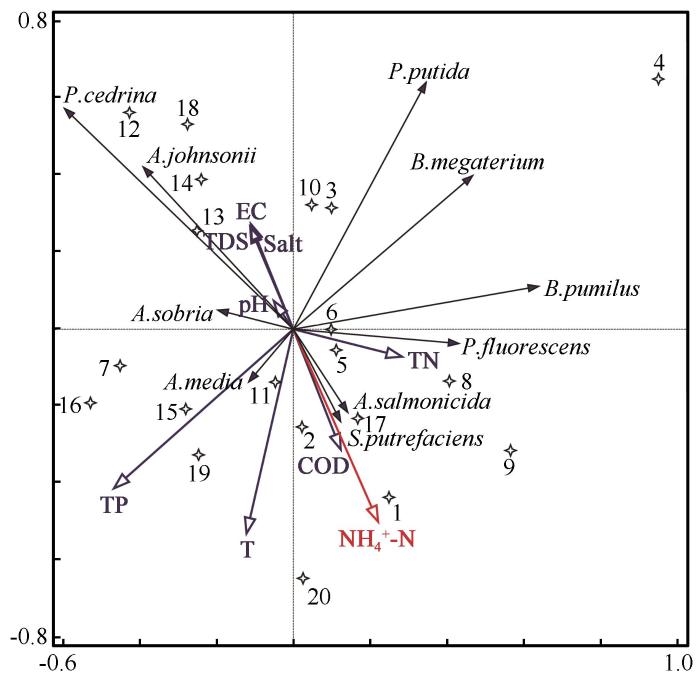
... 为更直观地反映细菌物种分布对于水质理化指标的响应规律,采用软件CANOCO 4.5对其进行排序分析.先进行除趋势对应分析,即DCA以确定合理的排序模型,DCA结果显示第一轴梯度长度值小于3,因此选择冗余分析,即RDA更加合理[20].在所有理化指标中,NH4+-N为对细菌群落分布具有显著影响的理化指标(P=0.03).为避免排序图杂乱,将低丰度物种排除后进行排序分析,图中仅展现相对丰度最高的10种细菌(图7). ...
纳木措春季沿岸水体可培养细菌群落特征
7
2022
... 2020年夏季沿纳木错设20个样点采集水样,本研究区域由于条件限制,无法达到湖面,因此仅在沿岸区设置样点.其中2号样点在扎西半岛,5号样点与主湖间隔了一道天然河坝,10号样点在多加寺附近,11号样点采样时水中长有水草,15号样点湖岸边上有很多动物粪便,19号样点在观景台,20号样点在湖泊入口处(图1).用无菌采集器在距湖岸边约15 m、距水体表面约50 cm处进行水样采集[20].直接在采样地用多参数测试笔测定水样pH、温度、总溶解固体量、电导率和盐度,每个参数测定3次[21].每个样点共采集约7.5 L水样,平均装入3个无菌塑料桶.1份水样立即送至西藏博源环境检测有限公司测定水体理化因子,包括总氮、总磷、氨氮、化学需氧量与浊度;1份水样带回实验室立即分离其中的可培养细菌[20]. ...
... [20]. ...
... 细菌的分离培养采用直接涂布平板法与稀释涂布平板法相结合.将20个样点的水样使用移液枪分别取200 μL接种于固体培养基平板(牛肉膏蛋白胨培养基:牛肉膏10 g,蛋白胨10 g,氯化钠5 g,琼脂粉20 g,水1 L,pH 7.4~7.6)上,采用涂布器将水样涂布均匀后放入恒温箱中倒置培养,温度设置为28 ℃.其中菌落数过多的样点重新采用稀释涂布平板法进行分离培养(5号、10号、11号、15号和19号).每个处理设3个重复.待平板上长出清晰可见的菌落时依次进行计数、分离、纯化及保藏等步骤,详见文献[20]. ...
... 对供试菌株16S rDNA序列测序结果进行人工校对,去除引物及双峰序列后在GenBank数据库中进行比对,从而确定菌株的分类地位[20].将供试菌株的基因序列上传至GenBank核酸数据库,登录号为MW799879~MW799953,共75条序列.下载与代表菌株基因序列相似性高的菌株序列,使用MEGA 7.0软件中的邻接(neighbour-joining)法构建基于16S rDNA基因序列的系统发育树,其中检验次数设为1 000次[23],确定本研究中菌株的系统发育学地位[20]. ...
... [20]. ...
... 各项理化因子的测定详见文献[20]. ...
... 为更直观地反映细菌物种分布对于水质理化指标的响应规律,采用软件CANOCO 4.5对其进行排序分析.先进行除趋势对应分析,即DCA以确定合理的排序模型,DCA结果显示第一轴梯度长度值小于3,因此选择冗余分析,即RDA更加合理[20].在所有理化指标中,NH4+-N为对细菌群落分布具有显著影响的理化指标(P=0.03).为避免排序图杂乱,将低丰度物种排除后进行排序分析,图中仅展现相对丰度最高的10种细菌(图7). ...
Diversity of culturable yeasts in Yamzhog Yumco Lake
1
2021
... 2020年夏季沿纳木错设20个样点采集水样,本研究区域由于条件限制,无法达到湖面,因此仅在沿岸区设置样点.其中2号样点在扎西半岛,5号样点与主湖间隔了一道天然河坝,10号样点在多加寺附近,11号样点采样时水中长有水草,15号样点湖岸边上有很多动物粪便,19号样点在观景台,20号样点在湖泊入口处(图1).用无菌采集器在距湖岸边约15 m、距水体表面约50 cm处进行水样采集[20].直接在采样地用多参数测试笔测定水样pH、温度、总溶解固体量、电导率和盐度,每个参数测定3次[21].每个样点共采集约7.5 L水样,平均装入3个无菌塑料桶.1份水样立即送至西藏博源环境检测有限公司测定水体理化因子,包括总氮、总磷、氨氮、化学需氧量与浊度;1份水样带回实验室立即分离其中的可培养细菌[20]. ...
羊卓雍措水体可培养酵母菌多样性及其与理化因子相关性
1
2021
... 2020年夏季沿纳木错设20个样点采集水样,本研究区域由于条件限制,无法达到湖面,因此仅在沿岸区设置样点.其中2号样点在扎西半岛,5号样点与主湖间隔了一道天然河坝,10号样点在多加寺附近,11号样点采样时水中长有水草,15号样点湖岸边上有很多动物粪便,19号样点在观景台,20号样点在湖泊入口处(图1).用无菌采集器在距湖岸边约15 m、距水体表面约50 cm处进行水样采集[20].直接在采样地用多参数测试笔测定水样pH、温度、总溶解固体量、电导率和盐度,每个参数测定3次[21].每个样点共采集约7.5 L水样,平均装入3个无菌塑料桶.1份水样立即送至西藏博源环境检测有限公司测定水体理化因子,包括总氮、总磷、氨氮、化学需氧量与浊度;1份水样带回实验室立即分离其中的可培养细菌[20]. ...
16S/23S rRNA sequencing
1
1991
... 菌株的鉴定采用16S rDNA基因序列分析法进行.本实验使用的上、下游引物序列如下:27F:5′-AGAGTTTGATCCTGGCTCA-3′;1492R:5′-GGTTACCTTGTTACGACTT-3′[22].聚合酶链式反应完成后采用1%琼脂糖凝胶检测PCR扩增产物,将其送至上海生工生物工程股份有限公司进行双向测序,各序列均测通. ...
Identification and characterization of yeasts isolated from sedimentary rocks of Union Glacier at the Antarctica
1
2016
... 对供试菌株16S rDNA序列测序结果进行人工校对,去除引物及双峰序列后在GenBank数据库中进行比对,从而确定菌株的分类地位[20].将供试菌株的基因序列上传至GenBank核酸数据库,登录号为MW799879~MW799953,共75条序列.下载与代表菌株基因序列相似性高的菌株序列,使用MEGA 7.0软件中的邻接(neighbour-joining)法构建基于16S rDNA基因序列的系统发育树,其中检验次数设为1 000次[23],确定本研究中菌株的系统发育学地位[20]. ...
Diversity and denitrification characteristics of culturable aerobic denitrifying bacteria in Dianchi Lake
2
2018
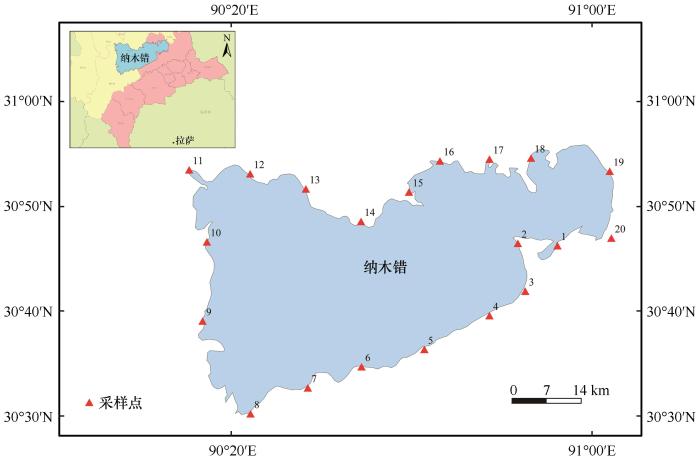
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
... [24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
滇池可培养好氧反硝化细菌多样性及其脱氮特性
2
2018
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
... [24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
Distribution of cold-tolerant bacteria and control of dominant bacteria in frozen chicken
1
2020
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
冷冻鸡肉中耐冷菌的分布及对优势菌的控制
1
2020
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
Screening and phylogeny of cryogenic strains producing protease from the bottom of the Tianshan No.1 Glacier
1
2013
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
天山一号冰川底部沉积层产蛋白酶耐低温菌株的筛选及其系统发育
1
2013
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
Analysis of bacterial diversity of chilled green shrimps under different packing methods
1
2019
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
不同包装方式下冷鲜青虾的菌群多样性分析
1
2019
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
Changes of bacterial composition and identification of dominant spoilage bacteria in squid during cryopreservation
1
2015
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
低温贮藏过程中鱿鱼细菌组成的变化及优势腐败菌鉴定
1
2015
... 从纳木错夏季水体20个样点中共获得681株细菌,分属于16属43种.本研究结果显示,Acinetobacter、Aeromonas与Pseudomonas为纳木错夏季水体细菌的优势属.王永霞等[24]从云南湖泊滇池中也分离到了这3个属,其中Pseudomonas为滇池的优势属,其次是Acinetobacter与Aeromonas,这3个属均为反硝化细菌属[24].张宇翔等[25]从三种冷冻鸡肉样品中分离到100株耐冷细菌,其中Pseudomonas占49%;倪永清等[26]通过对天山一号冰川底部沉积层耐低温菌的分离和其中产蛋白酶菌株的筛选,从125株分离物中筛选到27株产蛋白酶的耐低温菌株,其中Pseudomonas属菌株占40.7%,说明其具有较强的耐冷性.Acinetobacter[27]与Aeromonas[28]亦均可在低温条件下生存,因此较低的水温是Acinetobacter、Aeromonas与Pseudomonas在纳木错水体中分布广泛的可能原因之一. ...
Isolation and identification of specific putrid bacteria from cryopreserved Penaeus vannaei and analysis of putrid capacity
1
2016
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
低温贮藏南美白对虾特定腐败菌的分离鉴定及腐败能力分析
1
2016
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Research status of small molecule cold shock proteins in bacteria
1
2008
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
细菌中小分子冷休克蛋白的研究现状
1
2008
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Comparative study on characteristics of azo dye decolorization by indigenous decolorizers
1
2010
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Diverse responses to UV-B radiation and repair mechanisms of bacteria isolated from high-altitude aquatic environments
1
2006
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Research progress of Acinetobacter infection and drug resistance mechanism
1
2005
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
不动杆菌感染及耐药机制的研究进展
1
2005
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Acetylacetone-cleaving enzyme Dke1: a novel CC-bond-cleaving enzyme from Acinetobacter johnsonii
1
2003
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Reaction coordinate analysis for β-Diketone cleavage by the non-heme Fe2+-dependent dioxygenase Dke1
1
2005
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
A novel low-temperature alkaline lipase from Acinetobacter johnsonii LP28 suitable for detergent formulation
1
2011
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Characterization of novel diesel-degrading strains Acinetobacter haemolyticus MJ01 and Acinetobacter johnsonii MJ4 isolated from oil-contaminated soil
1
2012
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
The characteristics of nitrate removal by the psychrotolerant denitrifying bacterium Acinetobacter johnonii DBP-3, isolated from a low-temperature eutrophic body of water
1
2013
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Diversity change of microbial communities responding to zinc and arsenic pollution in a river of northeastern China
1
2014
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Intergeneric coaggregation of non-flocculating Acinetobacter spp. isolates with other sludge-constituting bacteria
1
2009
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Study on partial denitrification characteristics and kinetics of Acinetobacter johnsonii
1
2019
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
一株Acinetobacter johnsonii的部分反硝化特性及动力学研究
1
2019
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Screening of high lipase producing strain and analysis of its enzymatic properties
1
2019
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
高产脂肪酶菌株的筛选及其酶学性质分析
1
2019
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Thermostable ethanol tolerant xylanase from a cold-adapted marine species Acinetobacter johnsonii
1
2019
... A. johnsonii是纳木错夏季水体可培养细菌优势种,其在纳木错夏季水体中的分布和数量均具明显优势.有研究表明A. johnsonii是一种耐冷菌[29-30],因此,猜测其耐低温性是其在纳木错水体可培养细菌物种分布中占绝对优势的主要原因;且有研究表明A. johnsonii对于紫外线有一定抗性[31-32],而纳木错海拔高达5 000 m左右,紫外线强度高,因此,猜测这可能是导致其在物种分布中占绝对优势的另一原因.此外,有研究表明A. johnsonii具有多种生理生化作用,例如:A. johnsonii具有较普遍的耐药性[33];Straganz等[34]研究指出A. johnsonii具有二酮双加氧酶活性,而该酶能够催化乙酰丙酮分解反应,该物质对于动物有着神经毒性[35],因此A. johnsonii在降解有毒有害物质方面具有一定应用价值;Wang等[36]研究发现了1株具有产低温碱性脂肪酶活性的A. johnsonii,且该酶能够水解多种脂类;Lee等[37]研究发现A. johnsonii对于被污染的环境具备一定的生物修复功能;Li等[38]研究发现A. johnsonii有着一定的除磷能力;Zhao等[39]研究发现A. johnsonii能够生活在极度受污染的水体中;还有研究表明,A. johnsonii能够与其他菌株相互作用[40],以此来进行水体的生物修复;张阳等[41]研究发现A. johnsonii具有氮转化能力,有着较强的反硝化功能;张传丽等[42]研究发现A. johnsonii产脂肪酶活性较高,有一定应用价值;Xue等[43]从深海沉积物中分离出的一种产木聚糖酶的细菌菌株,该菌株被鉴定为物种A. johnsonii,其可以在低温环境下生长.总之,A. johnsonii应用价值突出,对于该菌株资源的开发及利用具有重要意义. ...
Bacterial community of the largest oligosaline lake, Namco on the Tibetan Plateau
1
2010
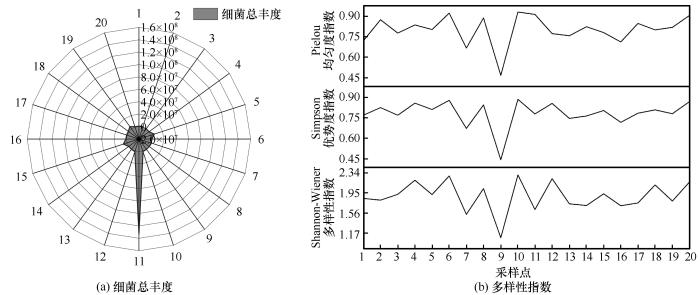
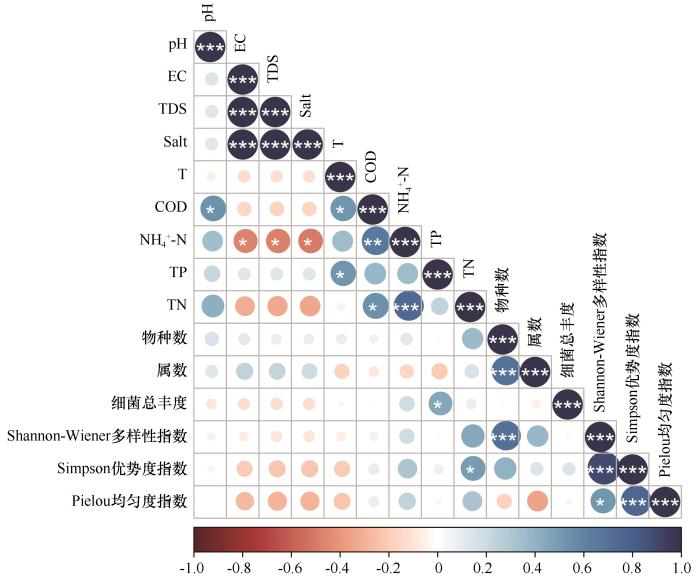
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Bacterioplankton community composition along a salinity gradient of sixteen high-mountain lakes located on the Tibetan Plateau, China
1
2006
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Bacterial community composition and diversity in Kalakuli, an alpine glacial-fed lake in Muztagh Ata of the westernmost Tibetan Plateau
1
2017
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Diversity and distribution of bacteria and archaea in Tuosu Lake in Qaidam Basin
1
2020
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
A comparison of pelagic, littoral, and riverine bacterial assemblages in Lake Bangongco, Tibetan Plateau
1
2014
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Spatio-temporal patterns of microbial communities and their driving mechanisms in Subalpine Lakes, Ningwu, Shanxi
1
2019
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Community composition and correlations between bacteria and algae within epiphytic biofilms on submerged macrophytes in a plateau lake, southwest China
1
2020
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Bacterial community changes in a glacial-fed Tibetan lake are correlated with glacial melting
1
2019
... 纳木错夏季各样点水体细菌多样性指数与水体理化因子相关性分析显示,TP是影响纳木错夏季各样点水体细菌总丰度的主要理化因子;TN是影响纳木错夏季各样点水体细菌多样性指数的主要理化因子.RDA结果显示,NH4+-N是影响纳木错夏季水体可培养细菌群落分布的主要环境因子.其他学者对高海拔湖泊细菌的研究中也有类似的结果.Liu等[44]曾指出细菌多样性的变化随着TN的浓度变化而起伏,Wu等[45]研究发现Salt是控制青藏高原东部海拔2 817~5 134 m的16个高山湖泊中细菌群落组成的主要环境因子,Liu等[46]发现TN对高原湖泊卡拉库里湖的细菌多样性具有重大影响,细菌群落组成与T和pH均显著相关,Song等[47]研究表明高原湖泊托素湖的细菌多样性和分布受到Salt和pH的高度影响.Liu等[48]研究发现高原湖泊班公湖细菌群落组成与TN的浓度密切相关.Wang等[49]研究发现NH4+-N是亚高山湖泊细菌群落的多样性的主要影响因素.Xia等[50]研究发现西南高原湖泊细菌的群落组成受T、NH4+-N、pH或TP的显著影响.Liu等[51]发现细菌的α多样性随季节而变化,并且与EC呈极显著的负相关. ...
Spatial dynamics of yeast community and its relationship with environmental factors in Lhalu Wetland, Tibet
1
2018
... 青藏高原地理位置及自然生境独特,该地区湖泊也蕴含着特殊的微生物资源,但高原湖泊生态系统由于极易受到外界影响及破坏,其中的微生物物种组成也易随之发生更替.湖泊水体细菌对于湖泊生态系统的变化具有响应规律,开展高原湖泊水体可培养细菌物种多样性的研究,不仅能够为极端环境下特殊细菌资源的开发利用提供理论参考,还能够为高原湖泊生态系统的保护提供理论依据[52]. ...
西藏拉鲁湿地水体酵母菌多样性及其与理化因子相关性
1
2018
... 青藏高原地理位置及自然生境独特,该地区湖泊也蕴含着特殊的微生物资源,但高原湖泊生态系统由于极易受到外界影响及破坏,其中的微生物物种组成也易随之发生更替.湖泊水体细菌对于湖泊生态系统的变化具有响应规律,开展高原湖泊水体可培养细菌物种多样性的研究,不仅能够为极端环境下特殊细菌资源的开发利用提供理论参考,还能够为高原湖泊生态系统的保护提供理论依据[52]. ...


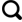



 甘公网安备 62010202000676号
甘公网安备 62010202000676号