目前冰盖约占地球陆地表面积的10%(在末次冰期冰盛期高达30%),冰储量的99%,但对其下的生物地球化学条件及其在极地生物地球化学循环中的重要性却知之甚少[1]。由于气候变暖,格陵兰及南极冰盖正在快速消融,过去十年里冰盖消融对全球海平面上升的贡献约为1 mm·a-1[2-3]。冰盖的冰下水文系统主要由饱和沉积物、冰下河及冰下湖泊等要素组成[4-8],它为极地生物地球化学风化速率的升高提供了有利条件[9-10]。微量元素(trace element,在天然水体中以较低浓度存在,通常<1 mg·L-1)作为地球化学风化的产物,其高产量与矿物表面高活性岩粉的大量补给有关,即物理侵蚀速率和冰-岩/沉积物界面处的岩水比率升高会使冰川磨蚀作用增强,进而产生大量岩粉补给,最终通过化学溶解作用释放出微量元素等[11-13]。冰盖经融水输出大量微量元素,是极地生物地球化学循环的重要组成部分[1]。
冰川融水被认为是下游水生生态系统中活性沉积物、溶质和营养盐的重要来源[14-17]。然而,针对冰川融水微量元素的研究却很少且主要集中在山地冰川流域[16,18-26]。尽管还不清楚许多微量元素的来源、形态、转化及其协同作用,但从微量元素浓度和形态等特征仍能获悉一些与化学风化环境、氧化还原过程、水体来源和冰下水文路径有关的重要信息,这些信息是无法通过离子化学获取的[27-29]。例如,Al和V可作为硅酸盐风化强度的示踪剂;Mo、Cd和Zn是强嗜硫元素,可指示硫化物矿物的氧化过程;Fe和Mn对氧化还原反应十分敏感,可指示源水的还原条件(还原条件会使Fe和Mn的氧化物溶解,导致其溶解浓度增加)和氧化速率大小;U主要源于沉积岩风化,其同位素可用作氧化风化作用的示踪剂;Sr可指示碳酸盐矿物的化学风化过程;一些微量元素(如Ni、Co、Pb和Cr)是强嗜铁元素,其流动性可以提供含铁矿物风化、元素输移以及通过缔合和生物介导的氧化还原过程等相关信息[1]。此外,许多微量元素(如Fe、Mn、Mo、Co、Zn、Cu、Cd和V)是生物必需的微量营养素,在细胞生长发育过程中发挥着重要作用,包括C、N和P的转运和同化以及作为代谢辅酶/金属蛋白的必要组分[30-31]。微量元素是地表生物地球化学过程的重要组成部分,作为维持生物生产力的微量营养素在碳循环中发挥着重要作用[1]。因此,经冰盖融水进入下游生态系统的微量元素可能会对生物生产力以及相关的营养限制作用和元素库存模式产生重要影响[16,32]。考虑到来自冰川流域的高融水通量,冰下独特的生物地球化学风化环境和逐渐升高的化学风化速率[9,33-35],量化冰盖中的微量元素输出对于了解下游元素循环和相关的生态系统响应以及未来冰川径流变化的潜在影响至关重要[1]。
近期,Hawkings等[1]指出,在冰盖的冰下环境中会产生具有较高生物地球化学活性的矿物微粒,这些微粒在微量元素循环中起着关键作用,并且对全球碳循环具有潜在的重要影响。具体来说,通过对南极冰盖(AIS)Mercer冰下湖水和格陵兰冰盖(GrIS)Leverett溢出冰川冰下融水进行采样,同时结合高分辨率电感耦合质谱仪/带有反应池的四极杆电感耦合质谱仪的测试分析,进而提供了冰盖融水中微量元素在不同粒径下的浓度数据[图1(a)]。通过这些数据:①阐释了生物地球化学风化过程和水源如何影响冰下环境的微量元素地球化学,并推测冰川衍生的微量元素对下游水生生态系统的潜在意义;②确定了所测大多数微量元素的胶体/纳米颗粒相的大小;③与全球非冰川河流平均值相比,评估了冰盖融水中微量营养素的富集/损耗。该研究结果显著促进了对冰盖生物地球化学过程的理解,并强调了冰盖融水在全球微量元素库存中的潜在重要性[1]。
图1
图1
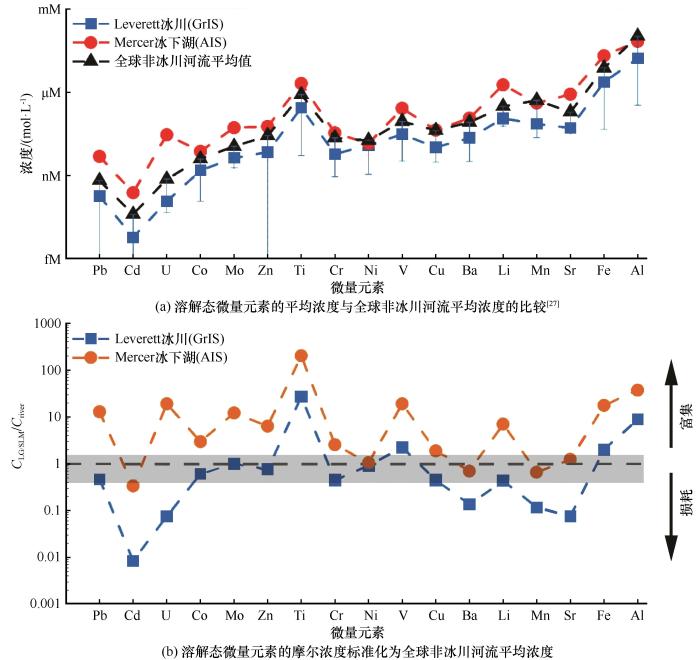
不同环境中溶解态微量元素的浓度对比[图(b)修改自文献[1];与全球非冰川河流相比,Leverett溢出冰川(LG)冰下融水和Mercer冰下湖(SLM)水中元素的富集/损耗情况表示方法为CNorm=CLG/SLM/Criver,将每种样品(CLG/SLM)的元素平均浓度标准化为非冰川河流(Criver)的元素浓度;数值大于1表示富集,小于1表示损耗;灰色区域表示河流平均值±50%]
Fig. 1
Comparison of dissolved (<0.45 μm) trace elements concentrations in various environments: dissolved mean elemental concentrations in meltwater compared to mean riverine concentrations[27] (a) and meltwater molar concentrations normalized to the global riverine mean dissolved elemental concentration (b) [Fig. (b) is modified from Reference [1]; The formula (CNorm=CLG/SLM/Criver) was used for normalization, where a value more than one equals enrichment and a value less than one indicates depletion, compared with mean riverine waters; The gray region indicates values ±50% of the riverine mean]
这项研究指出,与全球非冰川河流平均值相比[图1(b)],冰下环境中溶解态微量元素(<0.45 μm)的高浓度主要与矿物微粒的风化作用和融水在冰下排水系统中的滞留时间有关[1]。格陵兰和南极冰盖的融水中,来自硅酸盐和硫化物矿物风化作用的微量元素丰度表明,冰下环境中的生物地球化学风化过程可能是由硅酸盐和硫化物矿物的风化作用所主导,这与之前关于离子组成和矿物学的研究一致[9,35-37]。此外,高浓度的微量元素也反映了物理侵蚀过程的重要性,表现在产生了大量以原生矿物和纳米颗粒羟基氧化物矿物为主的具有较高生物地球化学活性的矿物微粒[1]。由于矿物微粒的吸附、表面沉淀和共沉淀作用,大多数溶解态微量元素主要以胶体/纳米颗粒相(0.02~0.45 μm)存在于冰下环境中[1],只有Sr、Mo和Li主要存在于可溶相(<0.02 μm)中,这可能与其在地表水体的高流动性有关[1]。因此,在确定冰下环境中溶解态微量元素的行为和浓度时,考虑胶体粒级至关重要。此外,冰盖融水中高浓度胶体/纳米颗粒态微量元素的存在可能会对其在下游的输移和不稳定性产生重大影响[1]。这些影响对Fe尤其重要,胶体/纳米颗粒态Fe会在低盐度下聚集、絮凝并从水柱中清除,这将对与Fe相关的微量元素产生影响[28]。
格陵兰及南极冰盖的水源具有不同的地球化学条件,因此其下发生的生物地球化学风化过程也有所不同。南极冰盖融水滞留时间较长,硅酸盐风化作用增强会产生大量黏土矿物,这些高度风化的沉积物在冰盖融水中可能发生表面离子交换[1]。这与格陵兰冰盖较短的融水滞留时间形成鲜明对比,即硅酸盐在新鲜破碎的原生矿物上发生快速水解和碳酸化反应(carbonation reaction,即在一定的压力和温度下,融水吸收二氧化碳形成碳酸的过程),并被冰面融水大幅稀释[1]。较长的融水滞留时间和相对隔绝的冰下水文环境似乎是南极冰盖溶解态微量元素浓度高于格陵兰冰盖的原因(高达一个数量级)。考虑到融水的滞留时间较长、底层沉积物的溶质输入[35]、未经地表融水稀释的冰下融水[14,36]等因素影响,一直认为南极冰盖环境中溶解态微量元素的浓度很高,但直到现在几乎没有证据支持。先前由于缺乏南极冰盖的地球化学数据,无法对冰盖下微量元素的分布规律、存在形式、控制因素等进行比较研究。目前从南极冰盖环境中获得的粒级微量元素浓度数据对理解冰盖消融在南大洋的施肥作用具有重要意义[1]。尽管已经认识到生物地球化学风化过程和水源对冰下环境微量元素地球化学的影响,但冰盖消融对极地生物地球化学循环的影响仍不了解[38]。
近年来,冰盖在极地生物地球化学循环中的重要性仍未受重视,并且在全球微量元素生物地球化学循环模型中被忽略。Hawkings等[1]的研究表明,冰盖融水可以提供一系列溶解态微量营养素[图1(a)],以维持冰盖周围沿海地区的生物生产力并通过对下游水生生态系统的施肥作用间接影响碳循环[39-40]。然而,在初级生产力较高的沿海地区,融水源微量营养素浓度较低,因此很难区分微量营养素的融水源及沉积物源[1]。显然,需要额外的观测资料和生物地球化学模型来区分微量营养素的具体来源,以及冰川通过直接(融水)和间接(沉积物)方式输入的影响。这凸显了冰盖对下游生产力影响的复杂性且目前很难量化。此外,这些微量营养素的最终归宿及其对海洋生态系统的影响将取决于物理和生物过程的复杂相互作用,包括冰前/湖泊/峡湾/河口的过滤效应(如底栖生物循环、矿物表面的吸附/解吸)、浮游植物的利用途径和营养限制等因素[13,16]。总之,由于微量营养素(尤其是Fe[28,41])的大量输出,未来需要将冰盖纳入微量元素的全球生物地球化学循环模型和邻近海洋系统的施肥研究中去。
厘清冰盖消融与极地生物地球化学循环之间的关系极具挑战性[38]。首先,相较于小型山地冰川,冰盖底部的融水样品难以获取[42],但对冰下湖泊等深层水环境的直接采样却是必不可少的,辅以地球物理方法可以从中推断冰盖底部的生物地球化学条件,这需要技术上的飞跃[13]。其次,相较于气候变暖,观测方案通常涵盖的时间周期较短,而且缺乏能够模拟冰盖生物地球化学过程及其更广泛影响的数学模型[43-45]。因此,未来将研究重点转向冰盖对下游水生生态系统的影响将有助于推动对极地生物地球化学循环的新认识,这应包括横跨整个陆地到海洋的连续性研究[13,38]。同时,建立耦合生物地球化学模型,包括冰冻圈(冰川与非冰川区)、大气和海洋之间的反馈,进而更好地评估冰盖消融对区域和全球微量元素生物地球化学循环的短期和长期影响[12]。此外,应将冰盖观测结果与最新最先进的生物地球化学耦合模型结合起来,这对解释冰盖周围沿海地区微量营养素的主要来源、过程和汇至关重要[13-14]。在未来,或许有助于理解冰期-间冰期的元素循环及其对气候系统的影响[1]。
参考文献
Enhanced trace element mobilization by Earth’s ice sheets
[J].
The land ice contribution to sea level during the satellite era
[J].
Pervasive ice sheet mass loss reflects competing ocean and atmosphere processes
[J].
Antarctic subglacial hydrology: current knowledge and future challenges
[J].
Recent advances in understanding Antarctic subglacial lakes and hydrology
[J].
Distribution and dynamics of Greenland subglacial lakes
[J].
The supply of subglacial meltwater to the grounding line of the Siple Coast, West Antarctica
[J].
Antarctic subglacial water: origin, evolution and ecology
[M]//
Weathering dynamics under contrasting Greenland Ice Sheet catchments
[J].
Glacier biogeochemistry
[J].
Rapid erosion beneath the Greenland Ice Sheet
[J].
Geochemical weathering in glacial and proglacial environments
[J].
Ice sheets matter for the global carbon cycle
[J].
The effect of warming climate on nutrient and solute export from the Greenland Ice Sheet
[J].
Storage and release of organic carbon from glaciers and ice sheets
[J].
Biogeochemical connectivity between freshwater ecosystems beneath the West Antarctic Ice Sheet and the sub-ice marine environment
[J].
Meltwater chemistry and solute export from a Greenland Ice Sheet catchment, Watson River, West Greenland
[J].
Diurnal hydrological-physicochemical controls and sampling methods for minor and trace elements in an Alpine glacial hydrological system
[J].
Climate versus geological controls on glacial meltwater micronutrient production in southern Greenland
[J].
Glaciers control the hydrogeochemistry of proglacial streams during late summer in the Wind River Range, Wyoming, United States
[J/OL].
Diurnal dynamics of minor and trace elements in stream water draining Dongkemadi Glacier on the Tibetan Plateau and its environmental implications
[J].
Seasonal controls of meltwater runoff chemistry and chemical weathering at Urumqi Glacier No. 1 in Central Asia
[J].
Elevated stream trace and minor element concentrations in the foreland of receding tropical glaciers
[J].
Diurnal hydrological controls and non-filtration effects on minor and trace elements in stream water draining the Qiyi Glacier, Qilian Mountain
[J].
Export fluxes of geochemical solutes in the meltwater stream of Sutri Dhaka Glacier, Chandra basin, Western Himalaya
[J].
Temporal and diurnal analysis of trace elements in the cryospheric water at remote Laohugou basin in northeast Tibetan Plateau
[J].
Trace elements in river waters
[J].
Ice sheets as a significant source of highly reactive nanoparticulate iron to the oceans
[J].
Molybdenum, vanadium, and uranium weathering in small mountainous rivers and rivers draining high-standing islands
[J].
Oceanic micronutrients: trace metals that are essential for marine life
[J].
The trace metal composition of marine phytoplankton
[J].
Processes and patterns of oceanic nutrient limitation
[J].
Globally elevated chemical weathering rates beneath glaciers
[J].
Chemical weathering under the Greenland Ice Sheet
[J].
Solute sources and geochemical processes in subglacial lake Whillans, West Antarctica
[J].
Investigation of subglacial weathering under the Greenland Ice Sheet using silicon isotopes
[J].
Ice sheets as a missing source of silica to the polar oceans
[J].
How does glacier discharge affect marine biogeochemistry and primary production in the Arctic?
[J].
Marine-terminating glaciers sustain high productivity in Greenland fjords
[J].
Environmental controls of marine productivity hot spots around Antarctica
[J].
Dissolved iron supply from Asian glaciers: local controls and a regional perspective
[J].
Environmentally clean access to Antarctic subglacial aquatic environments
[J].
Manganese in the West Atlantic Ocean in the context of the first global ocean circulation model of manganese
[J].
The role of external inputs and internal cycling in shaping the global ocean cobalt distribution: insights from the first cobalt biogeochemical model
[J].
Insights into the major processes driving the global distribution of copper in the ocean from a global model
[J].


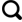



 甘公网安备 62010202000676号
甘公网安备 62010202000676号